Adoptive cell therapy, also known as cellular immunotherapy, is a form of treatment that uses the cells of our immune system to eliminate cancer.
Some of these approaches involve directly isolating our own immune cells and simply expanding their numbers, whereas others involve genetically engineering our immune cells (via gene therapy) to enhance their cancer-fighting capabilities.
Our immune system is capable of recognizing and eliminating cells that have become infected or damaged as well as those that have become cancerous. In the case of cancer, immune cells known as killer T cells are particularly powerful against cancer, due to their ability to bind to markers known as antigens on the surface of cancer cells. Cellular immunotherapies take advantage of this natural ability and can be deployed in different ways:
Today, cell therapies are constantly evolving and improving and providing new options to cancer patients. Cell therapies are currently being evaluated, both alone and in combination with other treatments, in a variety of cancer types in clinical trials.
Tumor-Infiltrating Lymphocyte (TIL) Therapy
Cancer patients have naturally occurring T cells that are often capable of targeting their cancer cells. These T cells are some of the most powerful immune cells in our body, and come in several types. The “killer” T cells, especially, are capable of recognizing and eliminating cancer cells in a very precise way.
The existence of these T cells alone, however, isn’t always enough to guarantee that they will be able to carry out their mission to eliminate tumors. One potential roadblock is that these T cells must first become activated before they can effectively kill cancer cells, and then they must be able to maintain that activity for a sufficiently long time to sustain an effective anti-tumor response. Another is that these T cells might not exist in sufficient numbers.
One form of adoptive cell therapy that attempts to address these issues is called tumor-infiltrating lymphocyte (TIL) therapy. This approach harvests naturally occurring T cells that have already infiltrated patients’ tumors, and then activates and expands them. Then, large numbers of these activated T cells are re-infused into patients, where they can then seek out and destroy tumors.
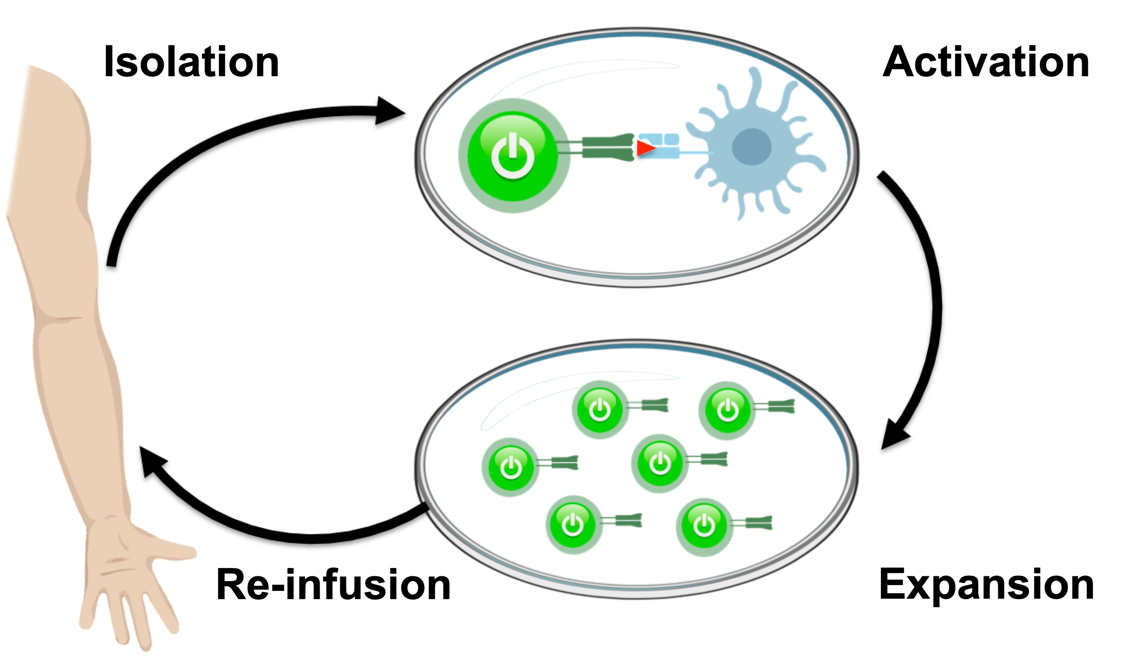
Engineered TCR Therapy
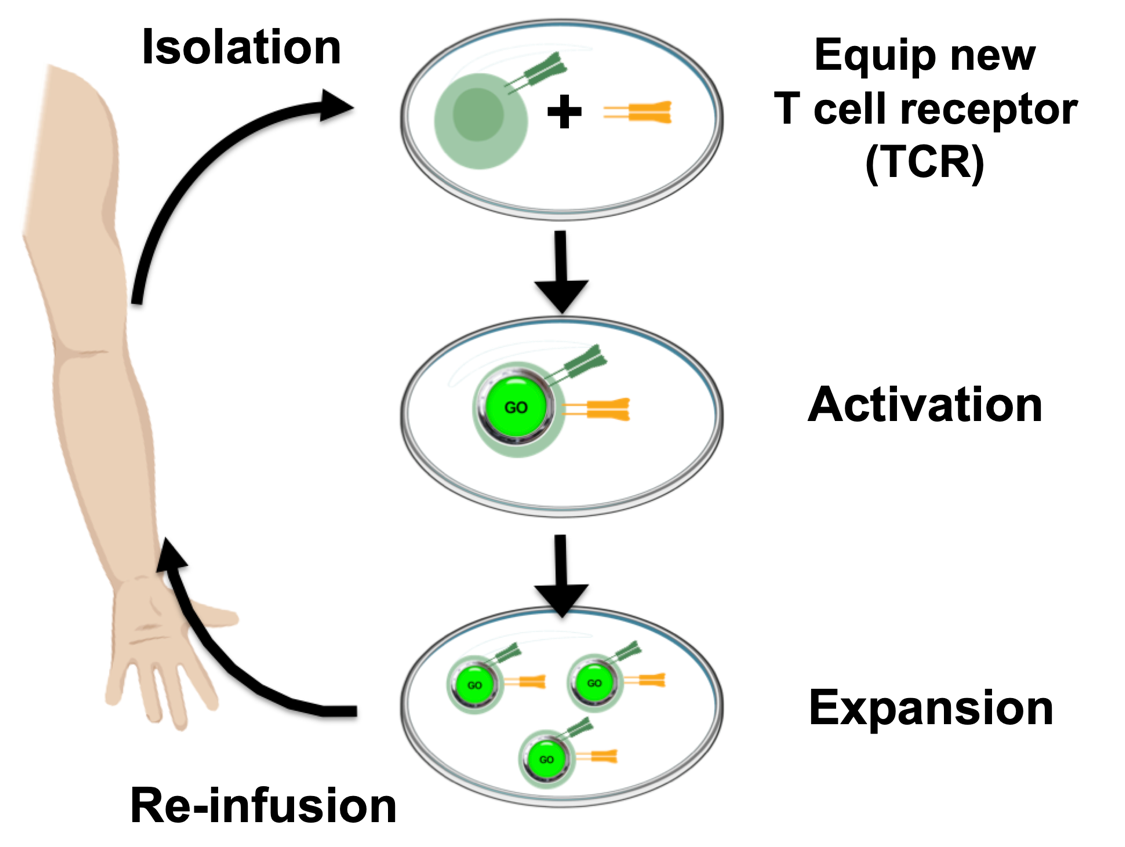
Unfortunately, not all patients have T cells that have already recognized their tumors. Others patients might, but for a number of reasons, these T cells may not be capable of being activated and expanded to sufficient numbers to enable rejection of their tumors. For these patients, doctors may employ an approach known as engineered T cell receptor (TCR) therapy.
This approach also involves taking T cells from patients, but instead of just activating and expanding the available anti-tumor T cells, the T cells can also be equipped with a new T cell receptor that enables them to target specific cancer antigens. By allowing doctors to choose an optimal target for each patient’s tumor and distinct types of T cell to engineer, the treatment can be further personalized to individuals and, ideally, provide patients with greater hope for relief.
CAR T Cell Therapy
The previously mentioned TIL and TCR therapies can only target and eliminate cancer cells that present their antigens in a certain context (when the antigens are bound by the major histocompatibility complex, or MHC).
Recent advances in cell-based immunotherapy have enabled doctors to overcome this limitation. Scientists equip a patient’s T cells with a synthetic receptor known as a CAR, which stands for chimeric antigen receptor.
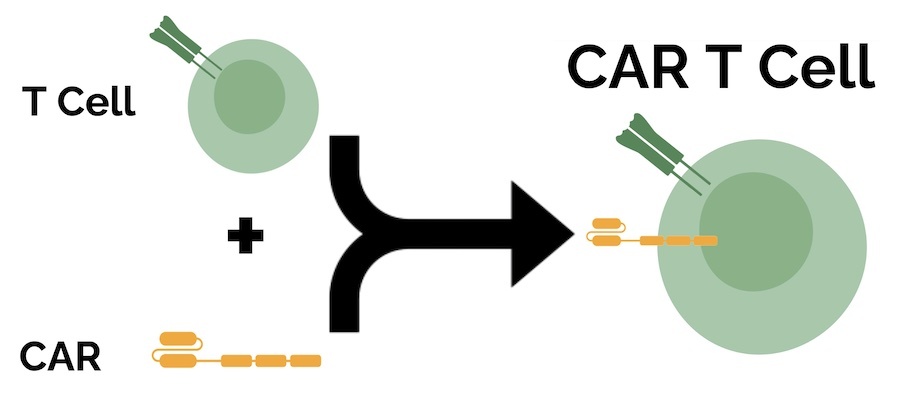
A key advantage of CARs is their ability to bind to cancer cells even if their antigens aren’t presented on the surface via MHC, which can render more cancer cells vulnerable to their attacks. However, CAR T cells can only recognize antigens that themselves are naturally expressed on the cell surface, so the range of potential antigen targets is smaller than with TCRs. In October 2017, the U.S. Food and Drug Administration (FDA) approved the first CAR T cell therapy to treat adults with certain types of large B-cell lymphoma.
Given their power, CARs are being explored in a variety of strategies for many cancer types. One approach currently in clinical trials is using stem cells to create a limitless source of off-the-shelf CAR T cells. This may have application to only selected settings, but could allow doctors to treat patients in a timelier fashion.
Natural Killer (NK) Cell Therapy
More recently, adoptive cell therapy strategies have begun to incorporate other immune cells, such as Natural Killer (NK) cells. One application being explored in the clinic involves equipping these NK cells with cancer-targeting CARs.
There are currently two adoptive cell therapies that are approved by the FDA for the treatment of cancer.
CAR T Cell Therapy
- Axicabtagene ciloleucel (Yescarta®): a CD19-targeting CAR T cell immunotherapy; approved for subsets of patients with lymphoma
- Brexucabtagene autoleucel (Tecartus™): a CD19-targeting CAR T cell immunotherapy; approved for subsets of patients with leukemia and lymphoma
- Ciltacabtagene autoleucel (Carvykti™): a BCMA-targeting CAR T cell immunotherapy; approved for subsets of patients with advanced multiple myelom
- Idecabtagene vicleucel (Abecma™): a BCMA-targeting CAR T cell immunotherapy; approved for subsets of patients with advanced multiple myeloma
- Lisocabtagene maraleucel (Breyanzi®): a CD19-targeting CAR T Cell immunotherapy; approved for subsets of patients with lymphoma
- Tisagenlecleucel (Kyrmriah®): a CD19-targeting CAR T cell immunotherapy; approved for subsets of patients with leukemia and lymphoma
Side Effects
Side effects may vary according to the type of adoptive cell immunotherapy—and what exactly it targets—and may also be influenced by the location and type of cancer as well as a patient’s overall health. Potential cell therapy-related side effects often take the form of an overactive immune response and may lead to excessive inflammation via cytokine release syndrome (also known as cytokine storm), and also to neurotoxicity from inflammation in the brain. Side effects can range from mild to moderate and may become potentially life-threatening under certain circumstances.
Fortunately, in most cases potential immunotherapy-related side effects can be managed safely as long as the potential side effects are recognized and addressed early. Therefore, it’s extremely important that patients inform their medical care team as soon as possible if they experience any unusual symptoms during or after treatment with cancer immunotherapy. In addition, patients should always consult their doctors and the rest of their care team to gain a better and fuller understanding of the potential risks and side effects associated with specific adoptive cell immunotherapies.
Common side effects associated with currently approved adoptive cell therapies may include but are not limited to: acute kidney injury, bleeding episodes, heart arrhythmias, chills, constipation, cough, cytokine release syndrome (cytokine storm), decreased appetite, delirium, diarrhea, dizziness, edema, encephalopathy, fatigue, febrile neutropenia, fever, headache, hypogammaglobulinemia, hypotension, hypoxia, infections, nausea, neurotoxicity, pyrexia, tachycardia, tremors, and vomiting.
Throughout its history, CRI has supported a variety of basic research projects aimed at improving our understanding of the identity and functions of our many immune cells as well as translational and clinical efforts that seek to use these insights in the development of cellular immunotherapies for cancer patients in the clinic.
Some of the most important contributions made by CRI scientists in the area of adoptive cell therapy include:
- In 1992-1995, CRI investigator Stanley Riddell, M.D., and CRI grantee Philip D. Greenberg, M.D., of the Fred Hutchinson Cancer Research Center, highlighted the importance of cytomegalovirus (CMV)-targeting T cells in protecting transplant recipients against life-threatening infections, and showed that adoptive cell therapy could restore CMV-specific immunity in transplant recipients.
- In 2002, CRI grantee Cassian Yee, M.D., along with Stanley Riddell, M.D., and Philip D. Greenberg, M.D., then all at the Fred Hutchinson Cancer Research Center, demonstrated one of the first successful uses of adoptive cell therapy in melanoma with patient-derived immune cells specifically targeting a tumor antigen.
- In 2003, CRI postdoctoral fellows E. John Wherry, Ph.D., and David Masopust, Ph.D., working in the Emory University lab of Rafi Ahmed, Ph.D., demonstrated the superior persistence and protective power of central memory T cells, and identified a receptor that was associated with these long-lived memory cells.
- In 2006, CRI postdoctoral fellow Yoshihiro Hayakawa, Ph.D., and Mark J. Smyth, Ph.D., of the Peter MacCallum Cancer Centre (Australia), revealed that Natural Killer cells can protect against tumor formation through the NKG2D pathway.
- In 2014, CRI investigator Michel Sadelain, M.D., Ph.D., of Memorial Sloan Kettering Cancer Center, showed that regional delivery of mesothelin-targeting CAR T cells improved their activity against cancer.
Current CRI grantees are working on a number of ways to improve adoptive cell therapy, including characterizing cellular exhaustion, evaluating innate-like T cells, designing new T cell receptors, and developing additional strategies to engineer T cells to improve their anti-tumor activity.