Immunotherapy for skin cancer provides treatment options for patients with advanced cases.
More people in the United States are diagnosed with skin cancer than all other cancers combined, with roughly 5.3 million new cases each year according to the most recent estimate, which projects one in five people in the U.S. likely to be diagnosed with skin cancer before age 70.
Fortunately, skin cancer is commonly diagnosed at an early stage when it can be dealt with more effectively. Only a small fraction—roughly 1 to 2 of every 500 cases—prove deadly.
The majority of skin cancers (90 percent) are linked with exposure to sunlight’s ultra violet rays, and as a result typically occur in sun-exposed areas such as the face, head, neck, arms, and legs, and are more common in those with lighter (less-pigmented) skin; however, skin cancers can occur in all people and can arise in areas that are rarely if ever exposed to the sun.
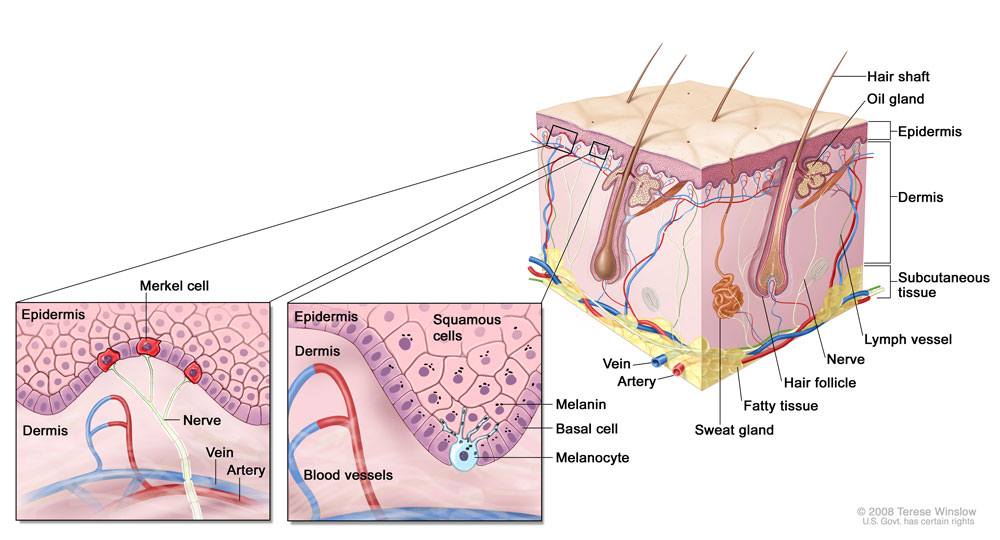
There are three main types of skin cancer, which arise in the three main types of skin cells:
- Basal cell carcinoma (BCC), which arises in the basal cells that produce new skin cells, is the most common type of skin cancer, comprising roughly 80 percent of all cases. Basal cell carcinomas rarely spread, or metastasize, to distant tissues.
- Squamous cell carcinoma (SCC), which arises in the squamous cells that form the skin’s inner lining, is the second most common type of skin cancer, comprising the vast majority of the remaining 20 percent of (non-basal cell) skin cancers. While metastasis occurs more frequently in SCC compared to BCC, it is still relatively uncommon but can occur if the disease is left untreated too long.
- Melanoma, which arises in the pigment-producing melanocytes, is much less common in the United States than both BCC and SCC, but is responsible for the majority of skin cancer deaths.
There are also several other types of skin cancer that occur much less frequently, including:
- Merkel cell carcinoma (MCC) is a very aggressive skin cancer that arises in Merkel cells and is often associated with the Merkel cell polyomavirus. MCC affects about 1,500 people each year in the United States.
- Kaposi’s sarcoma (KS) is a skin cancer that arises in the blood vessels of the skin and occurs mainly in people with weakened immune systems, such as people with AIDS or those who have received organ transplants and take immune-suppressing drugs to prevent transplant rejection.
While immunotherapy has been successful in melanoma and cutaneous squamous cell carcinoma, new treatment options for these and other skin cancers are urgently needed.
Subscribe to email alerts
Early-stage skin cancers that remain localized are often successfully treated through a variety of surgical techniques as well as radiation therapy, photodynamic therapy, and topical chemotherapy. For advanced cases beyond surgery, there are several chemotherapies and immunotherapies available for patients.
Immunotherapy is class of treatments that take advantage of a person’s own immune system to help kill cancer cells. There are currently twelve FDA-approved immunotherapy options for skin cancer.
Immunomodulators
- Aldesleukin (Proleukin®): a cytokine that targets the IL-2/IL-2R pathway; approved for patients with advanced melanoma
- Atezolizumab (Tecentriq®): a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved in combination with cobimetinib and vemurafenib for a subset of patients with advanced melanoma
- Avelumab (Bavencio®): a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with advanced melanoma and Merkel cell carcinoma (MCC)
- Cemiplimab (Libtayo®): a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with advanced cutaneous squamous cell carcinoma (CSCC) and advanced basal cell carcinoma
- Dostarlimab (Jemperli): a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with advanced skin cancer that has DNA mismatch repair deficiency (dMMR)
- Imiquimod (many brand names): an immune adjuvant targeting the Toll-like receptor 7 (TLR7) pathway; approved for subsets of patients with basal cell carcinoma
- Ipilimumab (Yervoy®): a checkpoint inhibitor that targets the CTLA-4 pathway; approved for subsets of patients with advanced melanoma, including as a first-line therapy and in combination with nivolumab
- Nivolumab (Opdivo®): a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with advanced melanoma, including in combination with ipilimumab
- Peginterferon alfa-2b (Sylatron®/PEG-Intron®): a cytokine that targets the IFNAR1 pathway; approved for subsets of patients with melanoma
- Pembrolizumab (Keytruda®): a checkpoint inhibitor that targets the PD-1/PD-L1 pathway; approved for subsets of patients with advanced Merkel cell carcinoma (MCC), melanoma, cutaneous squamous cell carcinoma (CSCC), and solid tumors with high microsatellite instability (MSI-H), DNA mismatch repair deficiency (dMMR), or high tumor mutational burden (TMB-H)
- Poly ICLC (Hiltonol®): an immune adjuvant targeting the Toll-like receptor 3 (TLR3) pathway; approved for subsets of patients with squamous cell carcinoma
Oncolytic Virus Therapy
- T-VEC (Imlygic®): a modified herpes simplex virus (HSV) that infects tumor cells and promotes their destruction; approved for subsets of patients with advanced melanoma
These checkpoint immunotherapy approvals were landmark events for the treatment of certain skin cancers. While the vast majority of cases of early-stage, non-melanoma skin cancer are successfully treated by dermatologists in an outpatient setting, those who have advanced disease that doesn’t respond to traditional treatment may want to consider clinical trials in which promising immunotherapy strategies are currently being evaluated.
Find a skin cancer clinical trial
Since its founding, the Cancer Research Institute (CRI) has dedicated numerous grants and fellowships to the research of skin cancer immunotherapy, primarily melanoma. Fortunately, many of these breakthroughs in melanoma have since benefited patients with other types of skin cancer, too. Recent CRI-funded discoveries and breakthroughs include:
- CRI CLIP Investigator John Carucci, M.D., Ph.D., discovered that blocking the activity of the JAK/STAT pathway reduces the growth of suppression-associated squamous cell carcinoma (SCC) tumors in mice, thus providing a potential strategy through which cancer patients who have also received organ transplants could be treated without compromising the tolerance of their transplants.
- Drew Pardoll, M.D., Ph.D., and Suzanne Topalian, M.D., Ph.D., members of the CRI-SU2C Dream Team at the Johns Hopkins University School of Medicine, revealed the benefits of anti-PD-1 checkpoint immunotherapy prior to surgery in patients with resectable Merkel cell carcinoma (MCC).
- The CRI Anna-Maria Kellen Clinical Accelerator is funding a clinical trial that seeks to bolster the anti-PD-L1 and anti-CTLA-4 combination immunotherapy through the use of an adjuvant designed to activate innate immune responses in various advanced accessible solid tumors.
Explore CRI’s current research into skin cancer in our funding directory.
Donate to skin cancer research