The COVID-19 pandemic has taken a toll on all aspects of daily life, disrupting routines, distancing loved ones, and causing many questions about safety amidst so much uncertainty. Health care is one area that was affected, with many people delaying regular health checkups or avoiding planned treatments due to concerns over COVID-19.
The implications of the COVID-19 pandemic for cancer incidence and mortality is not yet fully known, but researchers project that they will see some impact on the overall trends in cancer occurrence and deaths. One of the key areas to measure is the impact of COVID-19 on cancer clinical trials around the world. A team from the Cancer Research Institute (CRI) has been tracking this impact since the pandemic took hold in early 2020, and today has released a one-year follow-up to its earlier analysis, published in Nature Reviews Drug Discovery.
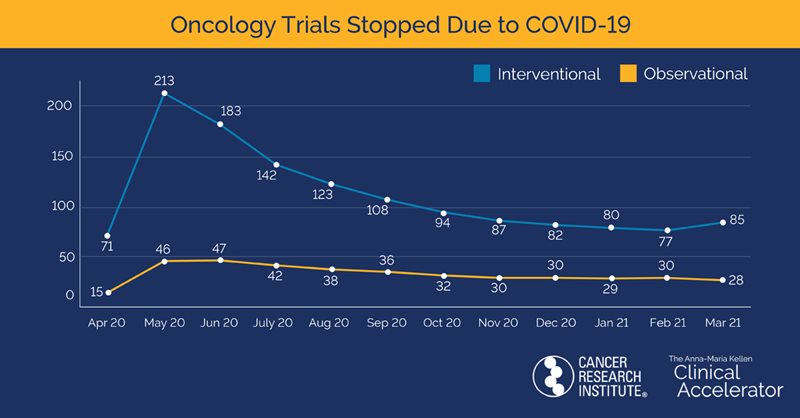
The newly published analysis from CRI revealed that the impact of the COVID-19 pandemic on oncology clinical trials globally has diminished, with more paused trials restarted at faster rates than non-oncology trials. The report also shows that this positive rate of reactivation was seen across interventional studies testing new therapeutic approaches regardless of tumor type or treatment type. In short, cancer clinical trials took less of a hit from the COVID-19 pandemic than trials focused on other health conditions and appear to be opening and accruing patients at increasing rates now compared to this time last year.
The CRI report shows that the number of clinical trials listed on clinicaltrials.gov that stopped due to COVID-19 peaked in June 2020, while the pausing or termination of oncology trials peaked one month earlier in May. A total of 386 oncology clinical trials were impacted with 274 reactivating (71%), 74 remaining paused, 27 terminating, and 11 being withdrawn. The percentage of trials reactivating contrasts starkly with the 1,665 non-oncology trials impacted by the pandemic, of which 665 reactivated (40%), 605 are still stopped, 287 were terminated, and 108 were withdrawn.
Not only was clinical research affected due to trial stoppage but patient care was also negatively impacted by the pandemic, with 1,155 planned oncology slots lost due to terminated and withdrawn studies. Lab closures and shifting priorities to COVID-19 likely delayed the progression of clinical trials. Nonetheless, most of the reactivated oncology trials were paused less than three months, with interventional studies restarting faster than observational studies. Oncology trials that did not resume operations during this period remain stopped. The 74 still paused oncology trials account for more than 99,940 planned patient enrollment slots. The type of therapy studied and cancer targeted do not appear to be factors in the reactivation of a trial. The majority of stopped oncology trials involved solid tumor cancers, with breast cancer accounting for 18% of these studies.
The findings from the Cancer Research Institute report suggest that the COVID-19 pandemic had less impact on the termination, withdrawal, and pause of oncology clinical trials than the pandemic had on non-oncology trials. Of note, the number of non-oncology clinical trials paused due to COVID-19 has risen slightly in the last five months, a sign that the world’s fight to contain this virus is not yet over. However, CRI analysts expect that the ongoing effect of the pandemic on all trials should lessen as the pace of vaccinations picks up globally.
Read the full findings from our one-year analysis in Nature Review Drug Discovery and access our up-to-date interactive dashboard on COVID-19-impacted studies.